Expanding our AI-driven diagnostics technology
Alleviating workflow challenges and the time it takes to read urine sediments.
This audio recording was enabled by AI
A fast and accurate diagnosis can enable a veterinary team to treat a pet quickly and effectively. And isn’t that what pet parents want? Fast, targeted treatments can help their dog or cat be as healthy and comfortable as possible.
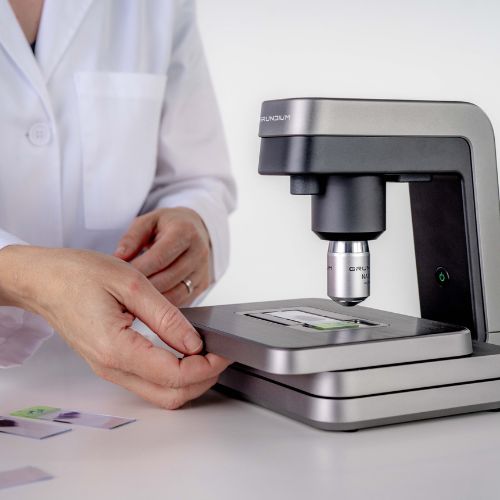
To equip veterinary practices with ever-better diagnostic tools, Zoetis continues to expand applications for Vetscan Imagyst®, a novel technology launched in 2020 that allows in-clinic testing through a single platform. The newest application is the AI Urine Sediment analysis. The application allows veterinary practices to analyze a pet’s fresh urine in the clinic and get results within minutes1, enabling quick and informed treatment decisions.1
In addition to AI Urine Sediment, the first-of-its-kind technology uses a compact scanner and artificial intelligence (AI) to deliver a variety of diagnostic tests, such as AI Fecal, AI Blood Smear, AI Dermatology and Digital Cytology. Additional applications are in development. Veterinary practices that use the technology also have access to a global network of expert clinical pathologists to provide additional support and consultation as needed.
“The addition of our newest application makes Vetscan Imagyst an even more powerful tool in the clinic,” said Dr. Richard Goldstein, a veterinarian and Vice President and Chief Medical Officer for Global Diagnostics at Zoetis. “Its deep learning AI will help in the visualization and identification of urine sediment elements, which are often difficult to capture using traditional urine sediment examinations.”
Vetscan Imagyst enhances the ability of the veterinary care team to perform vital screenings in the clinic within minutes, making it easier for timely, accurate treatment decisions for pets. And for pet owners, quick results can provide peace of mind that their beloved animal can receive the right care at the right time.
Read the full press release.
References
1Zoetis Data on file. Study report No. DHXMZ-US-23-218 (DX218)